美国食品和药物管理局日前授予辉瑞的新冠口服治疗药物紧急使用授权。据悉,这款名为Paxlovid的口服药是美国药监局授权的第一款专门用于对抗新冠病毒的口服抗病毒药物。
文章插图
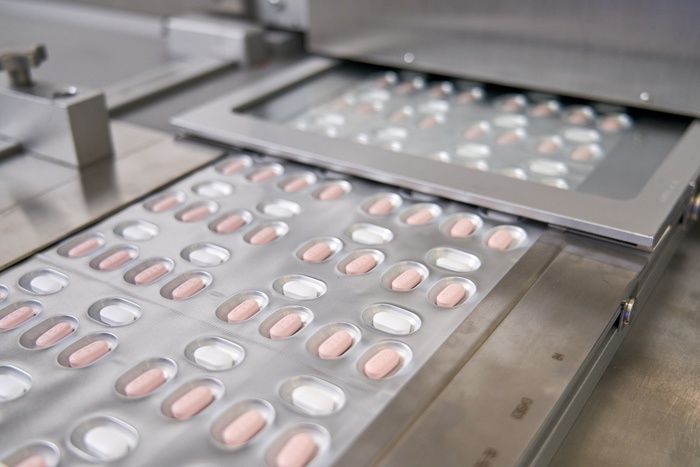
Paxlovid, a Pfizer's coronavirus disease (COVID-19) pill, is seen manufactured in Ascoli, Italy, in this undated handout photo obtained by Reuters on November 16, 2021. Pfizer/Handout via REUTERS
In a highly anticipated decision, the Food and Drug Administration authorized the first antiviral pill to treat COVID-19 at home.
在万众期待下,美国食品药品监督管理局日前批准了首款家庭用治疗新冠肺炎的口服抗病毒药物。
The pill, called Paxlovid, is made by Pfizer. It's taken twice a day for five days in combination with a second medicine called ritonavir, a generic antiviral.
这款名为Paxlovid的口服药是辉瑞公司生产的。该药须和抗病毒药物利托那韦一起服用,每日两次,一个疗程要连续服用五天。
"Today's authorization introduces the first treatment for COVID-19 that is in the form of a pill that is taken orally — a major step forward in the fight against this global pandemic," said Dr. Patrizia Cavazzoni, director of the FDA's Center for Drug Evaluation and Research. "This authorization provides a new tool to combat COVID-19 at a crucial time in the pandemic as new variants emerge and promises to make antiviral treatment more accessible to patients who are at high risk for progression to severe COVID-19."
美国药监局药物评估和研究中心主任派翠西亚·卡瓦佐尼博士表示:“首款新冠口服治疗药物今日获批,标志着我们迈出了抗击全球疫情的一大步,在出现新变异株的疫情关键时刻提供了抗击新冠的新工具,并承诺让新冠重症高风险人群更便于获得抗病毒治疗。”
The Pfizer treatment could help keep people infected with the coronavirus from getting so sick that they need to be hospitalized.
【 抗病毒药物|美国药监局批准首款新冠口服治疗药物 FDA authorizes 1st antiviral pill for COVID】 这款辉瑞口服药能有助于防止新冠病毒感染者发展成重症,避免住院治疗。
The results from a Pfizer study involving more than 2,200 people at high risk for developing serious COVID-19 found Paxlovid reduced the risk of hospitalization or death by 89%, compared with a placebo, when taken within three days of first symptoms of illness. When taken within five days, the drug reduced the risk of hospitalization and death by 88%.
一项涵盖了2200多名新冠重症高风险患者的辉瑞研究结果发现,相比安慰剂,患者在出现早期新冠症状三日内服用Paxlovid能将住院或死亡风险降低89%,在五日内服用能降低88%。
Early results from another Paxlovid study showed a 70% reduction in hospitalization risk among several hundred people at lower risk for severe disease.
另一项对Paxlovid口服药的研究涵盖了数百名新冠重症低风险患者,早期结果显示,可以将这一人群的住院风险降低70%。
Although it's not certain, Paxlovid's efficacy is unlikely to be reduced in treating people infected with the omicron variant of the coronavirus virus. The drug, which belongs to a family called protease inhibitors, doesn't target the virus's spike protein, as the vaccines do.
尽管尚不确定,但是用Paxlovid来治疗奥密克戎变异株感染者应该也同样有效。与疫苗作用的靶标不一样,Paxlovid是一种蛋白酶抑制剂,针对的不是病毒的纤突蛋白。
The federal government has a contract with Pfizer to buy 10 million courses of the treatment for $5.3 billion. But initial supplies of Paxlovid will be limited. The company says it will have 180,000 courses of treatment ready by the end of the year.
美国联邦政府已和辉瑞公司签约,以53亿美元(约合人民币338亿元)的价格购入1000万个疗程的药物。但是Paxlovid的初期供应量会比较有限。辉瑞表示,年底前将生产出18万个疗程的药物。
英文来源:美国国家公共广播电台
翻译&编辑:丹妮
- 日本首度公开立场轰动世界,美国彻底玩完,中国全面领先中···
- 酒精|医生的春节嘱咐:过年别喝酒,喝酒别吃药,5种药物“说走就走”
- 济南|济南2例确诊病例病情稳定,实施抗病毒、调解免疫等个性化治疗
- 亚硝基|美国全面禁售的食物,却让中国6000万人吃上瘾?看完后你还敢吃吗
- 医生|美国华人医生:接诊上万例奥密克戎,很多病人无发热和肺炎症状
- 呼吸道感染|今冬乙流冒泡 “高危密接”别忘药物预防
- 患者|辉瑞新冠口服药疗效好但美国人却很难得到
- 疗效|辉瑞新冠口服药疗效好 但美国人却很难得到
- 员工|美国医院要求染疫员工带病返岗 医护:这是在伤口撒盐
- 市场|报告:42 款中国手游 2021 年海外收入超 1 亿美元,美国为最大海外市场
